S-adenosylmethionine reacts with norepinephrine to form epinephrine – S-adenosylmethionine (SAMe) and norepinephrine (NE) embark on a captivating biochemical dance, leading to the formation of epinephrine, a hormone that orchestrates a symphony of physiological responses. This intricate interplay, orchestrated by the enzyme phenylethanolamine N-methyltransferase (PNMT), lies at the heart of our understanding of stress responses and metabolic regulation.
SAMe, a universal methyl donor, plays a pivotal role in numerous biological processes, including the methylation of DNA, proteins, and phospholipids. Its interaction with NE, a neurotransmitter and hormone, culminates in the synthesis of epinephrine, a molecule that exerts profound effects on the cardiovascular, metabolic, and immune systems.
S-Adenosylmethionine (SAMe) and Norepinephrine (NE)
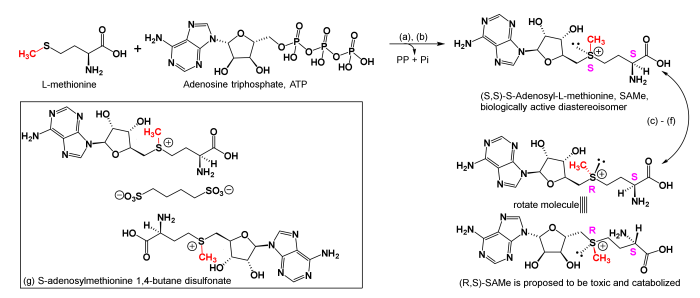
S-adenosylmethionine (SAMe) is a naturally occurring compound found in the body. It is involved in a variety of physiological processes, including methylation reactions, neurotransmitter synthesis, and cell growth and repair. Norepinephrine (NE) is a neurotransmitter that plays a role in mood, attention, and arousal.
SAMe and NE interact in the body to form epinephrine, another important neurotransmitter.
Chemical Structures of SAMe and NE
SAMe is a sulfonium compound with the chemical formula C 15H 22N 6O 5S. It consists of a methionine molecule with an adenosyl group attached to the sulfur atom. NE is a catecholamine with the chemical formula C 8H 11NO 3. It consists of a benzene ring with two hydroxyl groups and an amino group attached to it.
Physiological Roles and Interactions of SAMe and NE
SAMe is a methyl donor, which means it can transfer a methyl group to other molecules. This process is essential for a variety of cellular functions, including DNA synthesis, protein synthesis, and neurotransmitter synthesis. NE is a neurotransmitter that plays a role in mood, attention, and arousal.
It is released from the adrenal glands in response to stress.
SAMe as a Methyl Donor: S-adenosylmethionine Reacts With Norepinephrine To Form Epinephrine
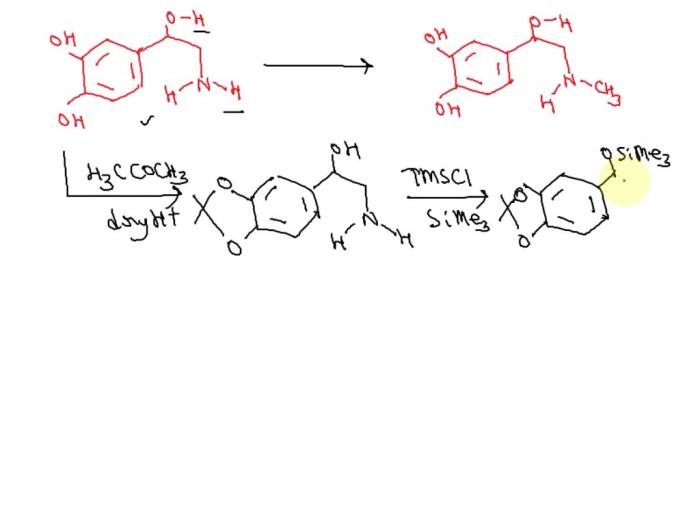
SAMe is a methyl donor, which means it can transfer a methyl group to other molecules. This process is essential for a variety of cellular functions, including:
- DNA synthesis
- Protein synthesis
- Neurotransmitter synthesis
- Cell growth and repair
SAMe donates its methyl group to a variety of acceptors, including proteins, lipids, and nucleic acids. This process is catalyzed by a variety of enzymes, including methyltransferases and transmethylases.
Formation of Epinephrine from NE
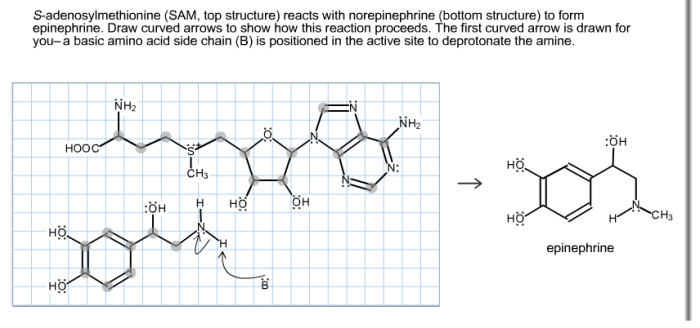
SAMe and NE interact in the body to form epinephrine, another important neurotransmitter. This reaction is catalyzed by the enzyme phenylethanolamine N-methyltransferase (PNMT). PNMT is found in the adrenal glands and other tissues.
The reaction proceeds as follows:
NE + SAMe → Epinephrine + SAH
where SAH is S-adenosylhomocysteine, a byproduct of the reaction.
Physiological Significance of Epinephrine, S-adenosylmethionine reacts with norepinephrine to form epinephrine
Epinephrine is a neurotransmitter that plays a role in mood, attention, and arousal. It is released from the adrenal glands in response to stress. Epinephrine causes the heart rate and blood pressure to increase, the airways to dilate, and the blood sugar levels to rise.
These effects help the body to prepare for a fight-or-flight response.
Regulation of Epinephrine Synthesis
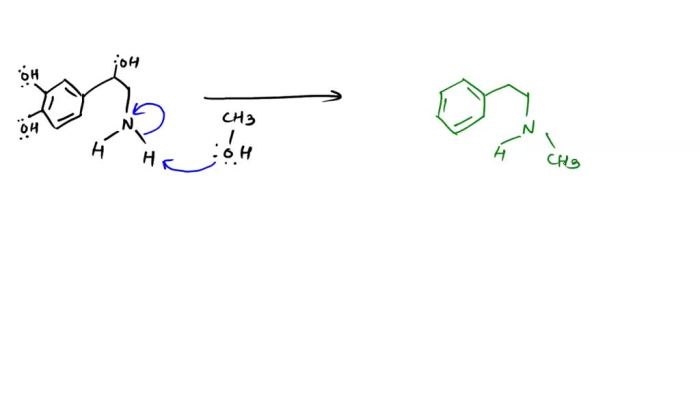
Epinephrine synthesis is regulated by a variety of factors, including:
- Stress
- Other stimuli that activate the sympathetic nervous system
- The availability of SAMe and NE
- The activity of PNMT
Stress and other stimuli that activate the sympathetic nervous system cause the release of NE from the adrenal glands. This NE can then be converted to epinephrine by PNMT.
The availability of SAMe and NE also affects epinephrine synthesis. SAMe is the methyl donor for the reaction, and NE is the substrate. If either of these compounds is not available, epinephrine synthesis will be decreased.
The activity of PNMT also affects epinephrine synthesis. PNMT is the enzyme that catalyzes the reaction between SAMe and NE. If PNMT is not active, epinephrine synthesis will be decreased.
Clinical Implications
SAMe has been shown to be effective in the treatment of a variety of conditions, including depression, osteoarthritis, and liver disease. It is thought that SAMe’s effects are due to its ability to increase the synthesis of neurotransmitters, such as epinephrine, dopamine, and serotonin.
SAMe is typically taken as a supplement, but it can also be given intravenously or intramuscularly. The usual dose of SAMe is 200 to 400 mg per day.
SAMe is generally safe and well-tolerated. However, it can cause side effects, such as nausea, vomiting, and diarrhea. SAMe should not be taken by people who are taking antidepressants or other medications that affect neurotransmitter levels.
Query Resolution
What is the role of SAMe in methylation reactions?
SAMe acts as a universal methyl donor, transferring its methyl group to various substrates, including DNA, proteins, and phospholipids.
How does SAMe interact with NE to form epinephrine?
SAMe donates a methyl group to NE, which is catalyzed by the enzyme phenylethanolamine N-methyltransferase (PNMT), resulting in the formation of epinephrine.
What is the physiological significance of epinephrine?
Epinephrine, also known as adrenaline, plays a crucial role in the body’s response to stress, preparing the body for a “fight or flight” response by increasing heart rate, blood pressure, and metabolic activity.